Many people asked me what technique I am using to photograph insects in ambers. The inspiration comes from Hellberg Jörgen, a Swedish photographer who on his website https://www.hellberg.photo/ describes the different results of photographing an amber with and without immersing it in a liquid.
The results are amazing!
The surface of amber is rarely perfectly flat and parallel to the subject you want to capture. Instead, it appears generally irregular or curved and, worse, it happens that the insect is closer to the lateral edge. This causes optical deformations when stacking the images, such as reflections on the surface, visibility of scratches, flaws, and fractures affecting the quality of the final image.
A method to obtain neat images and to make almost perfect results is to put the amber in a transparent or semi-transparent container (to facilitate the diffusion of light) and immerse it in a liquid that has approximately the same refractive index.
The best solution is to use glycerin. The refractive index of this liquid is 1.472, while that of amber is about 1.54; the very close values of the two substances make it possible to minimize the phenomena of light diffraction, hide the majority of scratches and fractures, and create the best working conditions for obtaining correct images. In the absence of glycerin (and a cheaper solution), sunflower oil can also be used, which has a refractive index between 1.472 and 1.476. You can also use immersion oil for microscopy (refractive index of 1.516, even closer to that of amber), but the price of the latter and the need to use a relatively important quantity (depending also on the size of the amber), make this technique relatively expensive. Finally, the use of a polarizer can help to eliminate any reflections present on the surface.
Keep in mind that amber has a density that is lower than that of glycerin, so it floats in this liquid. It is therefore necessary to fix the sample with plasticine (for example) on the bottom of the container and let the liquid flow until the sample is completely covered.

Caution should be exercised when pouring the glycerin because air bubbles may form between the bottom surface of the amber and the glass container; these are difficult to remove once formed. It is consequently necessary to remove the amber, dry it and use a new container.
Sometimes air bubbles may also form on the surface of amber, which should be removed with a needle and by using a stereomicroscope.
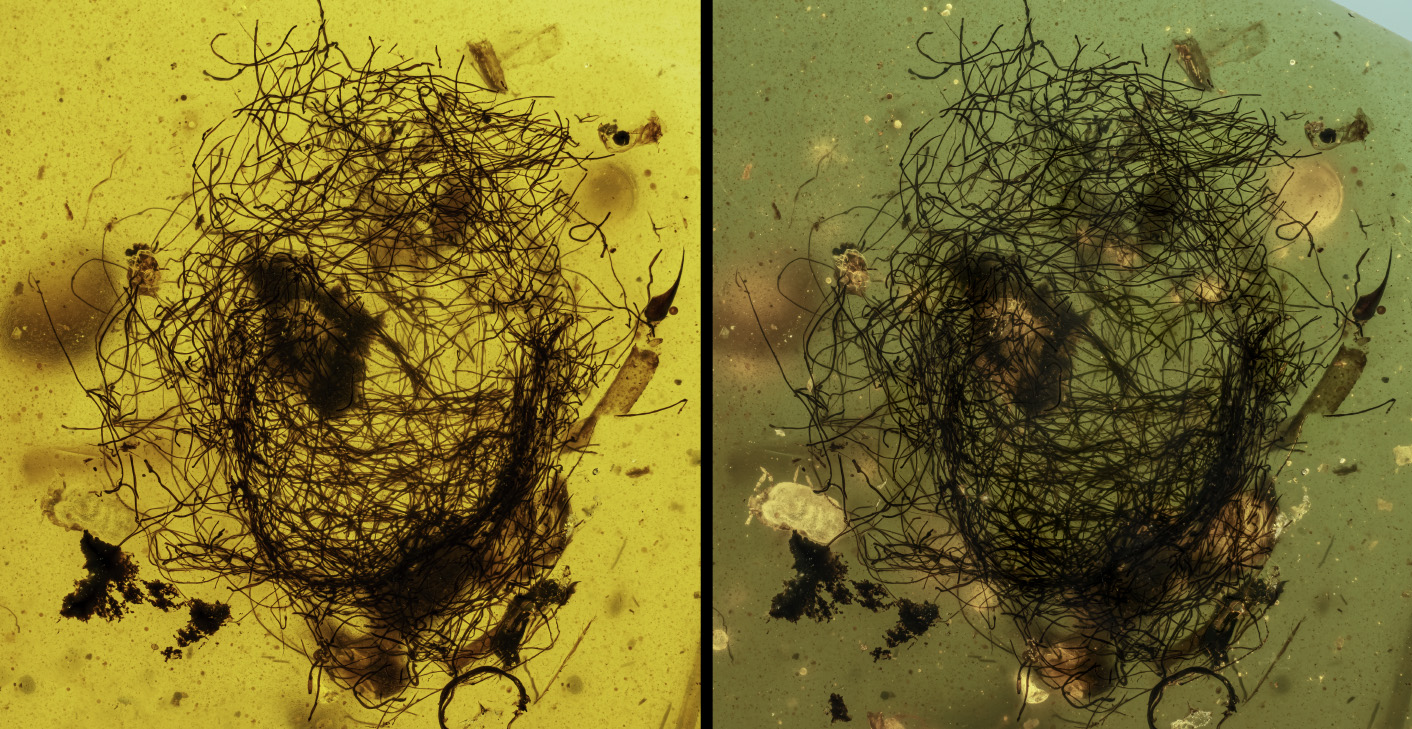
A recurring problem after multiple acquisitions have been made in glycerin is the presence of inclusions that float or move freely in the liquid. These are synthetic fibers that are present in the air or left behind by the fabrics used to dry the glassware or dropped accidentally. The slow movement is recorded in the frames you take, and the result often risks compromising the final photograph. In the example below, a beautiful (and rare) scorpion is “photobombed” by a hair that slowly and quietly crosses the field of view, leaving an irreparable trace that is impossible to remove.

This is an extreme (but not rare) case, but the judicious use of the tools available in Adobe Photoshop helps to resolve these unpleasant effects in many cases.
But what is the difference between an acquisition without and with glycerine?
In the two images below, you see a photo of a subject that is closer to the edge of amber with a hemispherical surface (the worst condition to obtain good images). I have used both images in the same lighting conditions and no corrections have been applied to improve the contrast, and the sharpness, eliminate the noise and remove the defects present on the surface.
Subject without immersion in glycerin:
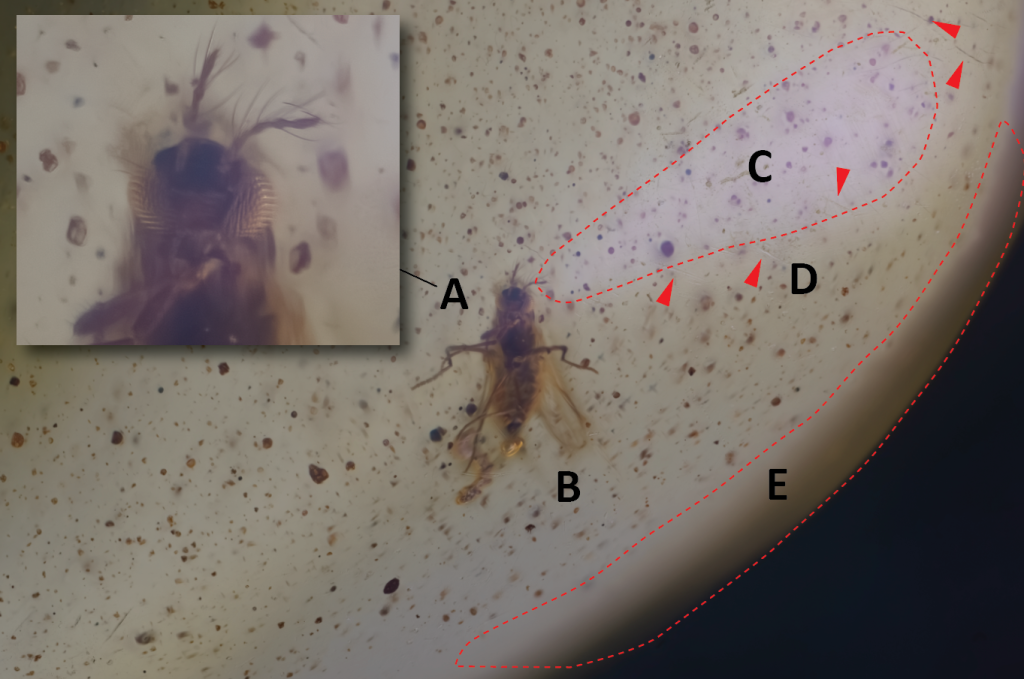
In addition to the poor image quality obtained (A), you can observe the deformation to which the insect is subjected when closer to the edge (B); a bright surface on the right side is the result of the reflection of the light coming from the LED panel, despite the presence of a diffuser (C); scratches, indicated by red arrows, are clearly perceptible on the surface (D); the details are lost near the outer edge (E).
Below is the subject immersed in glycerin:

The difference between the two results is even more visible by zooming in on the head and observing the details of the composite eyes, as visible in the insect.
The final image after processing.

This image is quite interesting because shows the presence of a bubble of air (a kind of “fart”) that protrudes from the anus. This bubble is due to the presence of bacteria inside the insect intestine, which continue their activity after the death of the insect.
Glycerol yes… glycerol no…
Particularly interesting argument! The use of glycerol as a liquid in which to dip ambers is used by some, and criticized by others, but it is not known the effect that this dense liquid of vegetable origin (it also exists in synthesis and animal derivation) has on ambers in the mid-long period.
As previously described, glycerol (glycerine is the commercial name which contains 95% of glycerol on it) has a refractive index close to that of amber, and this allows to remove of most of the optical deformations due to the non-flatness of the surface of amber (often smoothed and rounded) and eventual scratches.
To date, there is no scientific study that shows how in the mid-long-term ambers can be subjected to structural changes that deteriorate their surface and composition.
Alteration processes that affect amber
A recent paper published by Sadowski et al. (2021) details the factors that lead to the alteration process of ambers and their scientific importance. Amber is subject to constant mutation, more or less fast, depending on the age and the composition: the oldest ones would turn out, but not always, to be more resistant than the most recent ones, which crack and alter more easily (not to mention the Copal which is rubbish 😊 and extremely delicate). Sidorchuk (2013) and Sadowski et al. (2021) discuss some interesting techniques for protecting ambers by dipping them in epoxy resins and GST resin, but these methodologies require relatively expensive techniques and materials that are not readily available and need special caution. In addition, there is no knowledge of the med-to-long-term effects of these epoxy resins on amber and/or copal (Vincent et al. 2012).
The process of alteration of amber, as we said, is a fact, and every specimen that is brought to light, that is removed from its “natural” environment and matrix, begins a slow process of degradation, accelerated if subjected to changes in temperature, humidity, exposure to direct sunlight (the UV must absolutely be avoided, it is suggested to keep the samples in a dark environment), the action of oxygen (that cause the crazing and powder on the surface, and darkening), and the presence of pyrite. The latter during the process of natural alteration (called the pyrite disease), as well as giving rise to sulfuric acid and sulfate iron hydrates, increases in volume causing the formation of internal cracks that can extend up to the surface of the amber, breaking it.
Atmospheres contaminated with gases (ammonia, formic acid, acetic acid, sulfuric anhydride, formaldehyde, or ozone) and biocidal agents commonly used in museums (naphthalene, paradichlorobenzene, and camphor) also cause different degrees of cracking and deterioration on the surface of amber, although this deterioration seems to be due more to physical changes (expansion) than to chemical reactions (Corral, 1999).
From Corral (1999): “It is recommended that the specimens are kept in a non-fluctuating environment (relative humidity of 35-45% and a temperature of 16-20° C); and that they are maintained in darkness in the storage rooms or by filtering the ultraviolet radiation if they are kept in showcases.
Concerning long-term conservation, injection molding containers of polycarbonate or PET are preferable to those of polyethylene or polystyrene, since the degradation products that release the last, will eventually affect the fossil resin. The same can be said with respect to padding foams placed in the interior of the boxes. Knobs of acid-free paper and polyester felt have a more stable behavior than those of polystyrene foams.“

The phenomena of deterioration that are more relevant are the fracturing of the surface, the expansion of deeper fractures leading to fragmentation, and the change of color towards darker colors.
Generally, it is also indicated to avoid contact with chemical solvents that risk altering and dissolving the amber, unless you want to extract the fossil it contains to carry out more detailed studies, for example using Chloroform that can partly or dissolve completely the amber (Vincent et al. 2012). This operation is destructive and risks compromising the delicate fossil.

Finally, some publications mention that immersion in mineral and/or vegetable oils to take photographs is not recommended… but what about the use of Glycerol?
Glycerol …
Based on the bibliography I’ve been able to consult, there are no specific reasons or studies that clearly indicate that the use of this liquid is harmful to the amber itself. Quoting the recent work of Sadowski et al. (2021): “…some of the aforementioned substances (specifically glycerin, paraffin, and beeswax) can be difficult or impossible to remove…“. If paraffin and oils (mineral and vegetable) are difficult to remove, as they are not hydrophilic, a different matter should be made for glycerol, which is easily diluted in water and is just as easy to remove. It is a good rule to use distilled water to avoid the presence of impurities or mineral salts (mainly bicarbonates) that may deposit in the fractures, cavities and dull the surface.
The scientific publications that are generally referred to and that mention the subject (see bibliography below), are sometimes in favor of the use of glycerin. In Sidorchuk (2013) is stated that “Glycerol appears to be safe for Baltic, Rovno (Ukraine), Dominican Republic, Taimyr, and Burmese resins. It partly dissolves India amber (surface becomes tacky), so it must be applied with caution to “new” kinds of resin.“. Although the use of glycerol can be used for most ambers it is then recommended not to be used for ambers included in Class II (see Sadowski (2021), table 1) i.e., those originating from India, Arkansas, Zhangpu, and Copal (Sidorchuk, 2013; Sadowski, 2021). Other authors are vague or do not mention it at all, see Wunderlich (1983), Thikett (1995), Penney & Green (2010).
Finally, special attention should be given to fractured ambers; infiltration of fluids has led in some cases to loss of contrast and partial dissolution of the organism. In particular, this is visible in specimens where Canada balsam was used, as shown in some French Turonian (Cretaceous) ambers (Nel et al. 2020; Sadowski et al. 2021).
The use of vegetable oils such as sunflower oil, if not properly removed can be a fertile ground for the development of bacterial colonies and fungi, glycerol instead, if used concentrated and not diluted, inhibits the development of fungi and bacterial colonies: “The mechanism of action of glycerol is unknown, but it may make the substrate less accessible to the bacterial cells.” (Roger et al., 1992; Lind et al., 2010).
On the other hand, it turns out that glycerol despite being a potent inhibitor for cellular development, also turns out to be essential in cellular function when the presence of water is close to the limit for the existence of life itself (Stevenson et al., 2017), but here we are in the presence of extreme conditions anyway.
An interesting discussion on the effect of glycerol, and its osmolyte properties, on bacteria, is accessible here: https://chemistry.stackexchange.com/questions/46843/does-glycerin-promote-bacterial-growth-the-same-as-water . If at high concentrations it behaves like salt, absorbing water and leading to the death of bacteria, at low concentrations it is food for many microorganisms, promoting their reproduction and development.
And how does amber itself behave in presence of bacterial colonies? Amber has well-known antibacterial properties, and has been used for centuries in traditional medicine; today it is the subject of studies in the pharmaceutical sector (https://youtu.be/plUIPsoH7t4 ). Finally, anti-oxidative, antimicrobial, antiphlogistic, repellent properties and as an insecticide have been highlighted, see Tumiłowicz et al., (2016), and Al-Tamimi et al., (2020), but the bibliography is very vast.
As a conclusion to this short paragraph, we can state that there is no clear evidence that the use of glycerol can be harmful when in contact with amber; of course, one could also state that until proven otherwise, it is better to preserve amber from any (additional) alteration process.
Except for the possibility of being able to make acquisitions via electron microscopy, CT-scan, or cutting one’s own ambers in an optimal way in order to photograph them correctly (and in any case, an optical deformation would always be present), one of the best solutions for those who own a collection of ambers and want to share and preserve their memory, is to create a photographic archive of high-resolution images.
Finally, when stable environmental conditions for the preservation of specimens cannot be guaranteed, they can be stored in airtight plastic containers conditioned with silica gel (Silicagel, Art-sorb). In this way, independent and safe microenvironments can be created, provided, of course, that they are continuously maintained (Corral, 1999).

Image from https://doi.org/10.1016/j.crpv.2010.07.014
Improving the photographic quality
Following the photographic acquisition of a small insect preserved in amber from Myanmar (Upper Cretaceous, 99 million years ago), I was particularly struck by the insufficient quality of the stacked final image.
The subject is a small Staphylinidae (Coleoptera) having a size slightly larger than one millimeter, but the image (a result of a stack of 58 images, and acquired with an objective APO Mitutoyo 5x) appears to be affected by those typical effects of optical distortion that are visible in subjects that are not located near the surface of the amber, or when you are in the presence of layers with different density and optical characteristics.
But the amber is definitely small and the insect is located a couple of millimeters from the surface…

Hence the idea to try to further clean the surface, trying to abrade it with extremely fine glass paper, starting with 800 grit and then 4000 grit (yes maybe I exaggerated, but it works), and give a final polish with abrasive paste for the car body.

Wiping with a cloth also resulted in a much shinier surface than the original one.
The acquisition of a new series of images (with the same lighting conditions and acquisition times) has allowed verifying how the simple removal of just under half a millimeter of amber and better polishing has allowed obtaining a much more detailed image than the original image, as visible in the following images.

At left is the image before the polishing operation, and at right after the grinding and cleaning

At left is the image before the polishing operation, and at right after the grinding and cleaning
Then to reply to the question “How much can the thickness of amber between the subject and its surface affect the final photographic output?”, the answer is:
A LOT!
In the example below, I offer you two other subjects, acquired before (green background) and after (yellow background) the abrasion process with sandpaper (grit 1200 – 3000 – 4000 and cloth). It clearly emerges how, following the removal of part of the amber (~1mm), the details appear much sharper. The optical distortions visible in the composite eyes, antennae, thorax, and setae present on the wings of the subjects have been removed.

Above, a small Hymenoptera Ceraphronid (parasitic wasp)
Better results can be obtained by further processing the surface to within a few tenths of a millimeter of the insect. In this particular case, the presence of other syn-inclusions, and the need to preserve integrity, did not allow me to get any closer to the subject.
Hymenoptera, Chalcidoid, possibly closer to a Pteromalidae (Parasitic wasp)
The subjects are included in Burmese amber (Cenomanian, Upper Cretaceous ~99 million years ago).
Using colored background
The use of a white or black background allows one to highlight particulars of the subject that are more or less visible and aesthetic. For example, a black background will highlight the presence of iridescent colors from the internal amber fractures or internal foils of the beautiful golden colors, and a skillful orientation of the light source will help to increase the aesthetic part of the frame.
A white background, on the other hand, will highlight subtle structures such as localized bristles on the antennae, or hairs that occur on the wings, like the microtrichia, which are small and irregularly scattered, or the macrotrichia, which are larger, socketed, and generally restricted to the veins.


The use of light blue cardboard proves to be extremely beneficial in increasing the contrast between the subject and the surrounding matrix. As a result, a green or light blue coloration (depending on the thickness and color of the amber) is obtained as a background, while the subject presents a beautiful amber-yellow coloration that “stands out” better from the background.
The images that are shown below illustrate the results that can be achieved with this technique.

Osmylidae larva (Neuroptera) from Burma
In the case of the hairy structure below (animal or vegetal origin), notice how the mite located on the lower left side is much more visible using a blue cardboard background rather than a white background. Using a black background, on the other hand, would have reduced the visibility of individual hairs.

Latridiidae, Corticariinae from Baltic Area

Archeognatha from Dominican Republic amber
Essential Bibliography
Al-Tamimi W., Malik Al-Assadi S.A.A., Burghai A., (2020). Antibacterial activity and GC-MS analysis of Baltic amber against pathogenic bacteria. International Journal of Advanced Science and Technology, v.29(11s), pp. 611-618.
Corral, J.C. (1999). La conservación del Ambar. Revisión de los principales agentes de deterioro y soluciones publicados. Est. Mus. Cienc. Nat. de Alava, v.14(2), pp. 23-32
Lind H., Broberg, A., Jacobsson, K., Jonsson, H., Schnürer J. (2010). Glycerol Enhances the Antifungal Activity of Dairy Propionibacetria. International Journal of Microbiology, v. 2010, article ID 430873, 9 pp., doi:10.1155/2010/430873
Mikučionienė, D., Milašius, R., Daugelavičius, R., Ragelienė, L., Venslauskaitė, N., Ragaišienė, A., Rukuižienė, Ž. (2016). Preliminary investigation into the antimicrobial activity of an Electrospun polyamide nanofibrous web with micro particles of Baltic Amber. Fibres and Textiles in Eastern Europe. DOI: 10.5604/12303666.1215524
Nel, P., Schubnel, T., Perrichot, V., Nel, A. (2020). Ankothrips, the most ancient extant thrips genus (Thysanoptera, Melanthripidae). Papers in Paleontology, pp. 1-13.
Penney D., D.I. Green D.I. (2010). Introduction, preparation, study & conservation of amber inclusions. In D. Penney (Ed.), Biodiversity of Fossils in Amber from the Major World Deposits, Siri Scientific Press, Manchester (2010), pp. 5-12
Roger, V., Fonty, G., Gouet, P. (1992). Effects of glycerol on the growth, adhesion, and cellulolytic activity of rumen cellulolytic bacteria and anaerobic fungi. Current Microbiology, v. 25, pp. 197-201
Sadowski E-M., Alexander R. Schmidt, Leyla J. Seyfullah, Mónica M. Solórzano-Kraemer, Christian Neumann, Vincent Perrichot, Christopher Hamann, Ralf Milke, Paul C. Nascimbene, (2021). Conservation, preparation and imaging of diverse ambers and their inclusions, Earth-Science Reviews, v.220, 103653
Sidorchuk E.A. (2013). A new technique for the preparation of small-sized amber samples with application to mites. In D. Azar, M.S. Engel, E. Jarzembowski, L. Krogmann, A. Nel, J. Satniago-Blay (Eds.), Insect Evolution in an Amberiferous and Stone Alphabet, Proceedings of the 6th International Congress on Fossil Insects, Arthropods and Amber. Brill, Leiden (2013), pp. 189-201
Stevenson A., Hamill, P.G., Medina, A., Kminel, G., Rummel, J.D., Dijksterhuis, J., Timson, D.J., Magan, N., Leong, S-L. L., Hallsworth, J.E. (2017). Glycerol enhances fungal germination at the water-activity limit for life. Environmental microbiology, v.19(3), pp. 947-967
Thickett, D. P. Cruickshank, C. Ward (1995). The conservation of amber. Stud. Conserv., v.40, pp. 217-226
Tumiłowicz P., Synoradzki L., Sobiecka A., Arct J., Pytkowska K., Safarzyński S. (2016). Bioactivity of Balti camber – fossil resin. Polimery, v61(5), pp. 347-356
Vincent, G., Claudia, F., Solórzano Kraemer, M.M. (2012). Management of the Senkenberg amber collection and research developments. The Geological Curator, v.9(7), pp. 373-380/
Wunderlich J. (1983). Zur Konservierung von Bernsteineinschlüssen und über den Bitterfelder Bernstein. Neue Ent. Nachr., v.4, pp.11-13



